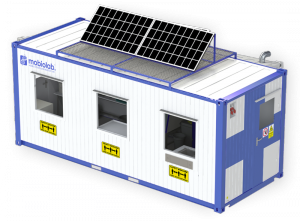
The first mobile container test facility for SARS-CoV-2
A Berlin-based startup has developed mobile test solutions for municipalities, hospitals, and companies
With the mobiolab, we want to support the incremental return to normalcy after the Corona crisis””
BERLIN, GERMANY, April 15, 2020 /EINPresswire.com/ -- Overview:— Dr. Rouven Soudry
mobiolab is a mobile plug-and-play laboratory solution for molecular biological diagnostics (using polymerase chain reaction, PCR)
Test procedure for SARS-CoV-2 and other pathogens
Significant test capacity of several hundred PCR analyses per hour at a fraction of normal costs
On-site increase in capacity and efficiency of medical, municipal testing and development of individual solutions for companies
With the mobiolab, the Berlin-based startup mobiolab GmbH offers the first mobile laboratory solution for the molecular biological diagnosis of SARS-CoV-2. The test laboratories are set up in freight containers and are ready for immediate use. They can operate self-sufficiently and offer PCR-based evidence of the virus genome – with the highest degree of sensitivity and specificity.
“With the mobiolab, we want to support the incremental return to normalcy after the Corona crisis”
Dr. Rouven Soudry, managing director of mobiolab GmbH.
“To master this massive undertaking, a mobile laboratory solution is a decisive component in expanding decentral capacities. We especially want to offer companies a reliable option to protect their business and employees.”
With a significant test capacity of several hundred PCR analyses per hour at a fraction of normal costs, additional test capacities can be provided to hospitals, laboratories, and public testing stations. And mobiolab offers companies the possibility of analyzing samples on site in less than 90 minutes, this can prevent a threatened shutdown due to infected personnel.
Maximum flexibility
The mobiolab is available in various configurations and does not rely on special companies, reagents, or equipment. This means it is equipped for all types of pathogens, even for various pathogens in future pandemics. It can be used for catastrophes as well as in daily operation for routine analyses and provides the highest degree of future reliability. A mobiolab can be transported by ship, plane, or truck. In this way, existing laboratories can expand capacities as needed, or new self-sufficient sites can be set up. This on-site analysis means there is no need to transport samples to a laboratory - an essential factor for the quality of the test material. The power supply uses a standard 230 V connection. An optional photovoltaic system enables self-sufficient operation; two lead-acid rechargeable batteries (emergency power supply) are ready for any emergency operation.
Economical monitoring of the environment and people
Depending on the test procedure used, the costs of an analysis are a fraction of standard market prices, which can exceed 200 euros per test. In the scope of environmental monitoring in the mobiolab, a qRT-PCR-based SARS-CoV-2 verification is possible for less than 10 euros a test.
Quick results with low material costs enable regular tests of employees or work surfaces, medical products, and transport media. Contrary to antibody-based tests, this technology offers an early detection of infections and contaminations. Evidence of an infection long before a person shows symptoms means preventative measures can be taken promptly. This ensures the continued operation of companies and public services and prevents business closures.
Molecular biological test procedure for SARS-CoV-2 and other pandemics
Corona viruses such as SARS-CoV-2 can be detected on the basis of their genetic material. Tiny amounts of genetic material on contaminated surfaces or a throat swab from a person is enough for a positive test result using polymerase chain reaction (PCR). Compared to immunological test procedures that aim for the proof of specific antibodies, and thus proof of having had the disease, the PCR can provide evidence of traces of the virus itself. Antibody-based quick tests are currently the subject of much discussion. However, along with the completely different approach, these generally require blood samples and are characterized by insufficient specificity, meaning there can be a positive result even if there is an infection with other, harmless Corona viruses.
The fluorescence-based qRT-PCR enables a quantification of the RNA levels and is the gold standard for test procedures. After a short preparation of the sample, the results are available within an hour. One single mobiolab unit, depending on equipment and test procedure, therefore, has the capacity to test from 1,500 to approximately 7,000 samples per day.
Dr. Rouven Soudry
mobiolab GmbH
+49 30 921038650
email us here
Visit us on social media:
Facebook
Twitter
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.